Medical Device Design Control Services
Toltec's design control services help companies comply with the design control requirements of FDA's CFR 820.30 and ISO13485:2003.
Gap Analysis. Let a Toltec Specialist provide a Gap Analysis on your Design Control System. For a fixed price we will:
• Review procedures for compliance to FDA 820.30 and current industry practices
• Review your Design History File(s) on selected projects
• Develop a summary report of findings, severity and priority of findings, as well as recommendations
Other Services. We offer hands-on document development for the following aspects of medical device design control:
• Project and V&V plans
• Creation of Design Input and Design Output documents
» Requirements specifications
» Design descriptions
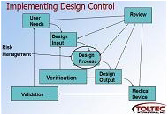 • Design Validation and Verification, including Software, including test protocols, test execution, and test records
• Design Validation and Verification, including Software, including test protocols, test execution, and test records
• Risk Analysis, FMEA Analysis, FTA Analysis
• Requirements Traceability
• Design Review records
• Design Control System and Procedure Development
Design Control Relation to FDA Submissions:
- Medical devices are subject to various regulations in terms of submission and approval (FDA)
- Design control deliverables are needed for the submission packages in many cases
Related links:
FDA's Design Control Guidance: http://www.fda.gov/cdrh/comp/designgd.html
Global Harmonization Task Force: http://www.ghtf.org/



 •
•